Undertaking human research at Mercy Health requires approval from the Mercy Health Human Research Ethics Committee (HREC). The Committee oversees human research activity at Mercy Health in accordance with the requirements of the National Health and Medical Research Council (NHMRC). Research can be submitted as being of either low risk or above– low risk. Low risk research can be submitted at any time. Research that is above-low risk must be reviewed by the full HREC at a meeting.
HREC submissions
The type of application and required documents are dependent on the level of risk of the proposed research.
- Low risk research may be reviewed by the Expedited Working Group, as delegated by Mercy Health HREC.
- Above-low risk research must undergo full review by Mercy Health HREC.
All Clinical Trial Principal Investigators are required to undertake research integrity training either through a face-to-face seminar or online module. PIs must include a certificate of completion with their submission to Mercy Health HREC.
Online research integrity training offered by the University of Melbourne is available to those with a UMelb staff/student number which can be accessed by clicking onto the training login link.
All new research projects must comply with REDCap Access and Use for Research Procedure to ensure the storage, use and disposal of research data and metadata protects the rights of individuals and maintains data security and integrity.
Low risk/negligible risk and quality assurance research
The National Statement on Ethical Conduct in Human Research 2007 (updated 2018) describes research as ‘low risk’ where the only foreseeable risk is one of discomfort. See NS chapter 2.1 for more information on low risk research.
Low risk research proposals can be reviewed by the Expedited Working Group. Applications for low risk research can be submitted at any time and are reviewed on a rolling basis.
Examples of low risk research:
- Data collection from existing records
- Questionnaire studies that do not involve personally intrusive questions, major time commitments or plans to publish information that could identify patients
Some quality assurance projects with only minor privacy concerns
Required low risk application forms
- Low Risk Peer Review Proforma
- Low Risk Application Form
- CV of Principle Investigator, or most experienced researcher, or study supervisor (2 pages, include contact details, education and previous research)
- If applicable, Quality Assurance Checklist * Please submit a completed checklist along with a summary of the project
Supporting documents (required where applicable to the project):
- Advertisements, media content
- Approvals from other HRECs
- Budget
- Data collection sheets
- Flyers, posters, leaflets
- Grant or funding information
- Letters to participants, phone scripts
- Protocol
- Participant Information and Consent Form (PICF), or Participant Information Statements (PIS)
- Section 2 of the Victorian Specific Module (when accessing medical records)
- Questionnaires, interview guides
Please note:
- Researchers must liaise with the Head of Department regarding their research and collect Head of Department/Divisional Director signatures before research proposal is submitted to the HREC Office
- The CMO signature will be collected by the HREC Office
- The complaints contact details for Mercy Health HREC have changed and are now: Feedback@mercy.com.au
Negligible risk and quality assurance projects
- Complete the Quality Assurance Checklist and submit along with a summary of the project.
Above-low risk research
Research which involves a risk of harm is considered above low risk research. Most clinical intervention research that involves the use of drugs or devices is considered above-low risk. All research applying an opt-out or waiver of consent process must be reviewed by the HREC in a meeting.
Examples of above-low risk research:
- Clinical trials involving new therapies and drug trials
- Major surveys or epidemiological studies requiring extensive data collection from patients
- Major studies involving the extraction of personally sensitive information from records
- Studies that involve vulnerable groups
- Research involving invasive procedures
All above-low risk research will be assigned a HREC facilitator who will review the project in advance and support the in-meeting review process.
Documents required for above-low risk research
| Document: | Description: | Access here: |
| Human Research Ethics Application (HREA) | The Human Research Ethics Application (HREA) form is an ethics application form developed by the National Health and Medical Research Council (NHMRC). | Ethics Review Manager |
| Victorian Specific Module (VSM) | The Victorian Specific Module has been designed so that the HREC can address Victorian specific legislative requirements. | Health.vic website |
| Site Specific Application (SSA) | Research governance application, required for site authorisation.
The SSA is created from the HREA on the Ethics Review Manager website. Governance authorisation requires signatures by the Research Governance Officer and CEO Health Services. Liaise with the HREC Administration Officer regarding these. |
Ethics Review Manager |
| Protocol | Required by Mercy Health HREC. | N/A |
Supporting documents (required where applicable to your project):
- Advertisements, media content
- Approvals from other HRECs
- Clinical Trial / Collaborative Research Agreements
- CVs for PI and researchers working on site at Mercy Health
- Data collection sheets
- Flyers, posters, leaflets
- Good Clinical Practice (GCP) certificate for PI
- Grant or funding information
- Indemnity agreement
- Insurance
- Letters to participants, phone scripts
- Participant Information and Consent Forms (PICF)
- Participant Explanatory Statements
- Questionnaires, interview guides
*Template PICFs are available here.
Additional documents for clinical trials:
- Instructions for participants
- Investigator brochure/product information
Original signatures are required on a Clinical Trial Research Agreement (CTRA) and indemnity documents.
More than risk process
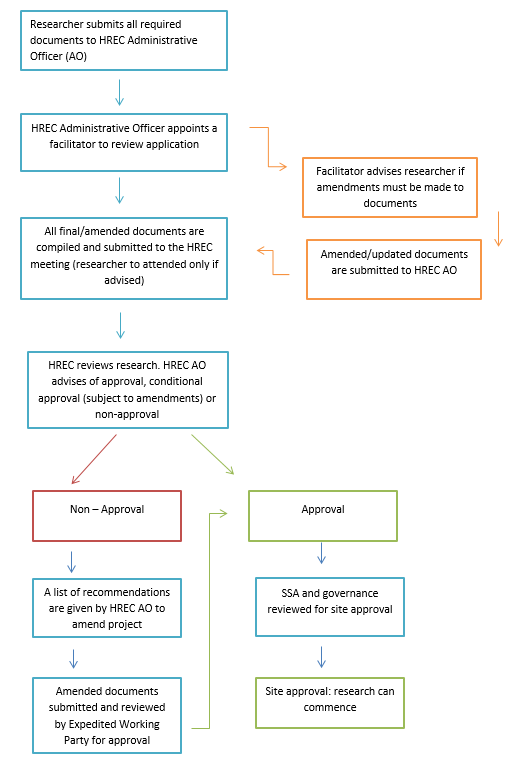
Collaborative research group clinical trials or investigator initiated clinical trials
A clinical trial must meet all governance requirements before it is approved to proceed. This includes evidence of, or clarification about, insurance, indemnity, sponsorship, funding, budgets, and trial registration number (CTN). The Principal Investigator (PI) or Site PI must contact the Mercy Health HREC Administrator prior to the submission date to discuss Mercy Health HREC application requirements.
Mercy Health HREC submission and meeting dates for 2024
| Submission Dates
Mondays |
Meeting Dates
Tuesdays |
| 19 December 2023 | 13 February 2024 |
| 26 February 2024 | 9 April 2024 |
| 22 April 2024 | 4 June 2024 |
| 24 June 2024 | 6 August 2024 |
| 2 September 2024 | 15 October 2024 |
| 21 October 2024 | 3 December 2024 |
| 30 December 2024 | 11 February 2025 |
After HREC approval
Once a research proposal has been approved other reporting will be required, including:
Progress Reports
- All approved research projects must submit an annual progress report, in accordance with the minimum reporting requirements of the National Health and Medical Research Council.
- Annual progress reports are due prior to the anniversary of the HREC approval date.
- Please download and complete the progress report form
- Please submit to ethics@mercy.com.au
Amendments
- Modifications to an approved project must first be approved by Mercy Health HREC. A template form is available to request an amendment.
- Download and complete the amendment request form.
- Amended documentation (such as protocols, investigator brochures, participant information sheets/consent forms) must be submitted showing tracked changes along with a clean updated version. A version number and date must appear on all documents.
- Please submit via email to: ethics@mercy.com.au
Serious adverse events
- A Site PI is responsible for reporting any Adverse Event (AE) or Severe Adverse Event (SAE)
- The Site PI must report the event to the HREC
- Please download from the Department of Health website.
- Please submit completed form to: ethics@mercy.com.au
Contact Mercy Health HREC
If you have questions regarding Mercy Health HREC requirements, please contact:
Administration Officer, Human Research Ethics Committee
c/- Mercy Hospital for Women
163 Studley Road Heidelberg, VIC. 3084
03 8458 4808
ethics@mercy.com.au
For further information on clinical trails, research and ethics requirements please visit health.vic.gov.au/about/clinical-trials-and-research
Last reviewed December 7, 2017.


